Novel Reactions
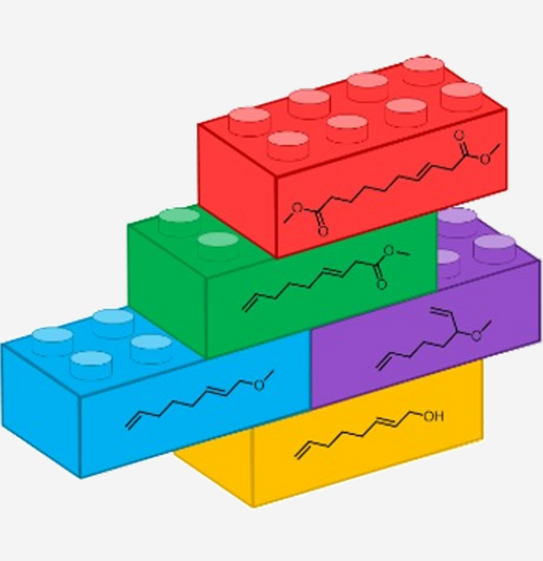
Direct Synthesis of an a,w-Diester from 2,7-Octadienol as Bulk Feedstock in Three Tandem Catalytic Steps
Ostrowski, K. A., Vogelsang, D., Seidensticker, T., Vorholt A. J.
Abstract: A new tandem catalytic process was designed and developed as at tool for the direct conversion of the widely available feedstock 2,7-octadienol into an a,w-diester. This innovative auto-tandem catalysis is atom efficient and consists of three consecutive palladium-catalysed reactions: ether formation, ether carbonylation and alkoxycarbonylation. By using the design of experiments (DoE) approach, significant parameters were determined and the yield of the desired a,w-diester was optimised. Model substrates allowed deeper insight into the progress of the reaction to be gained and, as a result, the reaction sequence was uncovered. Furthermore, by simply applying other ligands, a different reaction path was followed, allowing other, new tandem catalytic sequences to be explored and enabling new compounds to be obtained.
Chem. Eur. J. 2016, 22 (5), 1840-1846; DOI: 10.1002/chem.201503785
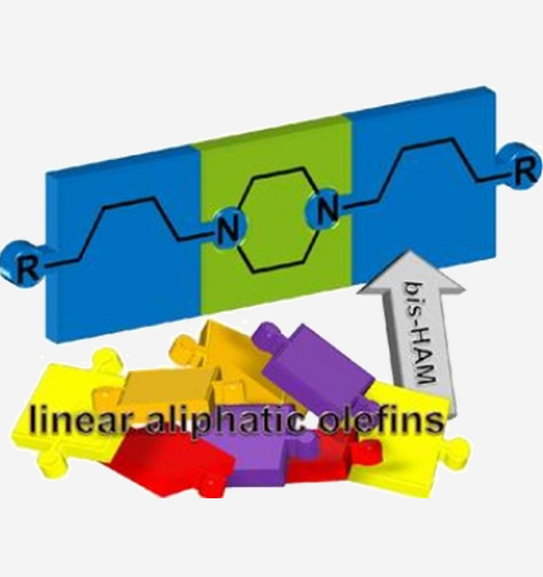
Rhodium-Catalyzed Bis-Hydroaminomethylation of Linear Aliphatic Alkenes with Piperazine
Seidensticker, T. , Vosberg, J. M., Ostrowksi, K. A., Vorholt, A. J.
Abstract: An efficient protocol was developed to prepare a series of dialkylpiperazines via Rh-catalyzed bis-hydroaminomethylation of linear aliphatic alkenes with piperazine. The well-known Rh/Biphephos catalytic system was applied, yielding the desired dialkylpiperazines within six tandem catalytic steps, already at low catalyst loadings of 0.1mol%. For the model alkene 1-octene, good yields and linearities of 80% and 77:23, respectively, were achieved under optimized conditions. Influences on the catalytic system regarding n/isoratio, possible side reactions and the reaction path are discussed on the basis of yield vs.time plots and parameter optimization. With the developed general protocol, other linear, functionalized and branched substrates were effectively transformed to the corresponding linear N,N-disubstituted piperazines.
Adv. Synth. Catal. 2016, 358, 610-621; DOI: 10.1002/adsc.201500896
Linear selective isomerization / hydroformylation of unsaturated fatty acid methyl esters –A bimetallic approach
Gaide, T., Bianga, J., Schlipköter, K. E., Behr , A., Vorholt, A. J.
Abstract: A key challenge in synthesis of non-ionic surfactants is opposite polarity of the substrates and the connected challenge to use homogeneous catalysis. We present the telomerisation ofβ-myrcene with N-methylglucamine to C20-N-alkylated polyols, which show surface activity. The use of aqueous solvent systems along with amphiphilic ligands bridges the polaritiy gap and shows high reactivities.
ACS Catal. 2017, 7, 6, 4163-4171; DOI: 10.1021/acscatal.7b00249
![Mensch mit Cap mit der Aufschrift [Pd] hilft Mensch mit Cap mit der Aufschrift [Rh] über eine Mauer die mit "Isomerization" und "Hydroformylation" und der Strukturformel von einer ungesättigten Fettsäuremethylester beschriftet ist](/storages/tc-bci/_processed_/3/6/csm_NovelReactions3v3_f552480661.png)
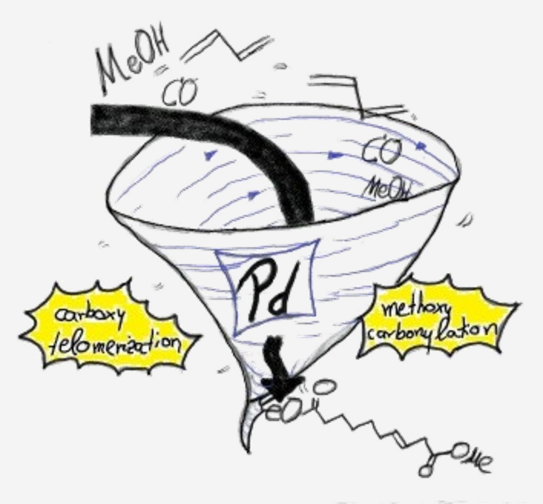
From Carboxytelomerisation of 1,3-Butadiene to Linear a,w-C10-Diester Combinatoric Approaches for an Efficient Synthetic Route
Vogelsang, D., Raumann, B. A., Hares, K., Vorholt, A. J.
Abstract: Two novel reaction pathways were tested to synthesize the linear a,w-C10-diester exclusively from three basic reagents: 1,3-butadiene, carbon monoxide and methanol. Therefor, carboxytelomerization of 1,3-butadiene with methanol was merged with methoxycarbonylation in two different ways to obtain highly linear C10-diester. Through a palladium-based and –assisted tandem catalytic system, 22% yield of the desired C10-diester was obtained without isolating the intermediates. Subsequently, the limitations of the novel assisted tandem catalytic concept were uncovered and based on that, a two-step reaction regime was established. By optimization of the carboxytelomerization, the C9-monoester as intermediate could be formed in nearly quantitative yields and excellent linearity. In a second reaction step, the isolated monoester was successfully converted by methoxycarbonylation into the desired linear C10-diester in overall yields up to 84%.
Chem. Eur. J. 2018, 24, 9, 2264-2269; DOI: 10.1002/chem.201705381