Dr. Kevin Hares
Contact through the Secretariat:
monika.boschtu-dortmundde

Curriculum Vitae
Kevin Hares started studying chemistry at TU Dortmund in October 2013 and finished his bachelor thesis with the topic “Development of a Tandem-catalytic Conversion of Butadiene into C10-unsaturated Esters” in 2016.
He continued with the masters at TU Dortmund, but went to the UK in September 2017 and studied at University College London for 9 months via the Erasmus+ exchange program. At UCL he completed a research project with the topic “Kinetic Studies on SSZ-13 and Synthesis of Hierarchical SSZ-13 using Chitosan as Macro-Template”. During this time and further until March 2019 Kevin received the “Deutschlandstipendium” scholarship by BASF SE.
In 2019 he finished his masters with a thesis on “The Palladium Catalyzed Carboxytelomerization of 1,3-Butadiene with Carboxylic Acids - An Atom Economic Route Towards Mixed Anhydrides” at the chair of industrial chemistry (TC).
Since April 2019 Kevin works as a research associate at the laboratory of Industrial Chemistry.
Research Topic
The modern chemical industry requires sustainable transformations of basic feedstock to be more cost efficient and less pollutive. A novel approach for this is the use of catalysts, which can be able to lower the activation energy of a specific reaction pathway and therefore lead to highly selective and active reaction systems.
Some reactions, which require similar catalysts and reaction conditions, can be carried simultaneously in a single reactor. If the same catalyst catalyzes these reactions, the reaction is called tandem catalysis depicted in figure 1.
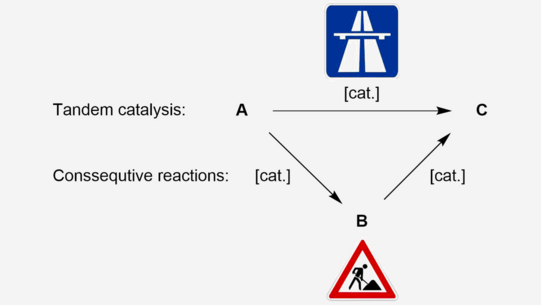
Conventional consecutive reaction require multiple steps and often a work up procedure between each step. These require energy and other resources, which can be saved in tandem-catalyzed reactions, because in this case the reaction system is able to convert A directly to C in one reaction set up (figure 1). Therefore, this “fast track” rout saves energy, time and resources.
Amination reactions and catalyst design
The synthesis of amines and especially primary amines is still challenging and subject of current research. One prominent route towards amines is the amination of alcohols. However, the selectivity of this reaction is difficult to control, since the resulting non-tertiary amines can undergo unwanted consecutive reactions.
A novel route for the synthesis of amines would be the amination of esters, since they are readily available. Additionally esters can be provided through renewable sources such as plant oil, hence this route can be based on a renewable feedstock. This reaction set up can be carried out in two steps or in a tandem-catalyzed manner as depicted in figure 2.
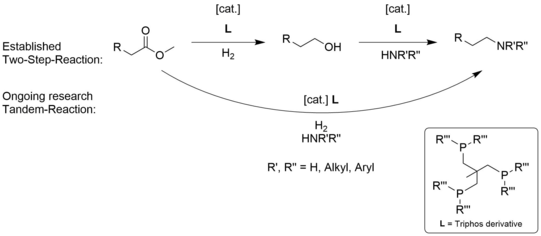
The mechanism of this reaction is not understood in detail, therefore it is of interest to determine key reaction steps and intermediates. This can be achieved through variation of the catalyst system. In addition, tailored ligands can increase the productivity of the system or optimize its selectivity. However, tailored ligands are not commercial available in most cases and have to be synthesized, which is an important part of this research.
Carboxytelomerization – A useful tool in Synthesis
Carboxytelomerization in another example for a highly atom economic tandem catalyzed reaction (figure 3). It is capable of transforming basic starting material into valuable unsaturated products, such as unsaturated esters. The reaction is well understood using alcohols as starting material such as methanol. However, recent studies have shown the flexibility of the reaction.

For example, it was possible to convert amines and branched dienes into their corresponding esters and amides (figure 4). The scope of the reaction can still be diversified and result in new valuable products efficiently to expand the synthesis-tool carboxytelomerization.

Publications & Conferences
- Hares, K., Wegener, H. W., Roth, T. F. H., Reichert, R., Vogt, D., Seidensticker, T., (2024) "Primary amines from alkenes and carbonyl compounds: highly selective hydrogenation of oximes using a homogeneous Ru-catalyst" Catal. Sci. Technol., Advance Article, DOI: 10.1039/D4CY00368C.
- Hares, K., Vogelsang, D., Wernsdörfer, C.S., Panke, D., Vogt, D., Seidensticker, T. (2022). „Palladium-catalyzed synthesis of mixed anhydrides via carbonylative telomerization” Catal. Sci. Technol., 12, 3992-4000, DOI: 10.1039/D2CY00486K .
- Vogelsang, D. , Vondran, J. , Hares, K. , Schäfer, K. , Seidensticker, T. , Vorholt, A. J. (2019). "Palladium Catalysed Acid-Free Carboxytelomerisation of 1,3-Butadiene with Alcohols Accessing Pelargonic Acid Derivatives Including Triglycerides under Selectivity Control". Adv. Synth. Catal. 362 (3), 679-687, DOI: 10.1002/adsc.201901383.
- Vogelsang, D. , Raumann, B. A. , Hares, K. , Vorholt, A. J. (2017). "From Carboxytelomerization of 1,3‐Butadiene to Linear α,ω‐C10‐Diester Combinatoric Approaches for an Efficient Synthetic Route". Chem. Eur. J. 24 (9), 2264-2269, DOI: 10.1002/chem.201705381.
- March 2021, Weimar, Germany, 54. Jahrestreffen Deutscher Katalytiker, "Expanding the synthesis tool carboxytelomerization-More efficient amide synthesis and new products"





