M. Sc. Jonas Vosberg
Contact through the Secretariat:
monika.boschtu-dortmundde

Curriculum Vitae
| 1985 | Born in Kempten, Germany |
| 2006 - 2013 | Bachelor of Science, TU Dortmund University, "Tandem-catalytic synthesis of monomer building blocks" (Prof. Arno Behr) |
| 2013 - 2016 | Master of Science, TU Dortmund University, “Nickel-catalyzed linear dimerization of isobutene” (Prof. Arno Behr and Dr. Andreas J. Vorholt) |
| 2017 - 2020 | Doctorate in Chemistry (evaluation pending), TH Cologne[1] and RWTH Aachen[2], “heterogeneous bases in aldol addition reactions” (Prof. Regina Palkovits[2] and Dr. Matthias Eisenacher[1]) |
| 2019 | certified 6σ Green Belt |
| Since 2020 | Post-Doc, TU Dortmund University, (Prof. Dieter Vogt) |
Research Topic
The profitability of an industrial scope chemical process depends, among other factors, on the availability and pricing of the chemical feedstock and the energy demand of the whole process. This can be achieved by establishing reactions based on a reliable and unproblematic feedstock such as CO2 and by combining multiple reactions into one single step. By doing so, this research aims at proving the feasibility of an alternative reaction route to an amide that itself is considered a large-scale platform chemical.
The research is carried out in close partnership with a globally renowned chemical cooperation.
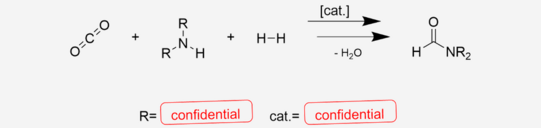
The investigated reaction is the CO2 hydrogenation to formic acid in the presence of a low molecular weight amine and the consecutive condensation of the acid and the amine to an amide. The latter step is an equilibrium reaction. This study includes the investigation of the limits that the chemical equilibrium applies to the maximum yield and strategies to overcome those boundaries.
Publications & Conferences
-
Vosberg, J. M., Drönner, J., Lummerich, F., Eisenacher, M. (ongoing proceeding): Aldoladdition – Verfahren zur Synthese von Aldoladditionsprodukten, Patent AZ 19-001, PROvendis AZ 5604 19.
- Seidensticker, T., Ostrowski, K. A., Vosberg, J. M., Vorholt, A. J. (2016). "Rhodium Catalyzed bis-Hydroaminomethylation of Linear Aliphatic Alkenes with Piperazine". Adv. Synth. Catal. 358 (4), 610-621, DOI: 10.1002/adsc.201500896.
- Behr, A., Rentmeister, N., Möller, D., J. M., Peitz, S. (2014). "Dimerization reactions with iron and cobalt bis(imino)pyridine catalysts: A substrate based approach". Catal. Commun. 55 (5), 38-42, DOI: 10.1016/j.catcom.2014.06.003.
- Behr, A., Rentmeister, N., Seidensticker, T., Vosberg, J., Peitz, S., Maschmeyer, D. (2014)."Highly Selective Dimerization and Trimerization of Isobutene to Linearly Linked Products by Using Nickel Catalysis". Chem. Asian J. 9 (2), 596-601, DOI: 10.1002/asia.201301263.
- March 2020, Weimar, Germany: DECHEMA 53. Jahrestreffen Deutscher Katalytiker: "Lithium Zirconate as a Replacement for Homogeneous Bases in Aldol Additions".
- March 2019, Weimar, Germany: DECHEMA 52. Jahrestreffen Deutscher Katalytiker: "From acetaldehyde to 1,3-butanediol - revising an industrial classic".
- December 2018, Leverkusen, Germany: STEPsCon 2018, ”From acetaldehyde to 1,3-butanediol - revising an industrial classic“.





