Dr. Till Köhler
Research Topic
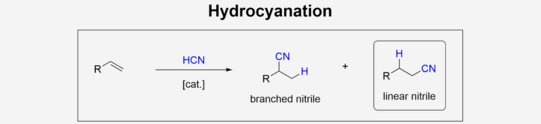
In chemical Industry, nitriles are versatile products featuring a various follow-up chemistry e.g. amines through hydrogenation or even Amides/Carbonic acids by hydrolysis. Homogeneous catalyzed addition of hydrogen cyanate to olefins can be used to produce nitriles. This so-called hydrocyanation of olefins offers great benefits over more traditional synthesis like Kolbe-nitrile synthesis, that is based on nucleophilic substitution. The low reactivity as well as moderate selectivity towards the linear nitrile while using non-activated olefins like 1-hexene, remains challenging in the application of hydrocyanation in chemical industry.
A novel approach in ligand design for increased catalysts activity while keeping high regioselectivity is highly promising.
Publications
-
Köhler, T., Rienhoff, B., Vogt, D., (2024) "Nickel(BiPhePhos)-Catalyzed Hydrocyanation of Styrene—Highly Increased Catalytic Activity by Optimized Operational Procedures" Catalysts, 14(3), 210, DOI: 10.3390/catal14030210.
- March 2023 Dortmund, Germany, 11th Young Chemists Symposium Ruhr 2023, "Hydrocyanation of unbiased alkenes by enhanced Nickel-Lewis-acid-cooperativity and investigation of the influence of promotors"





